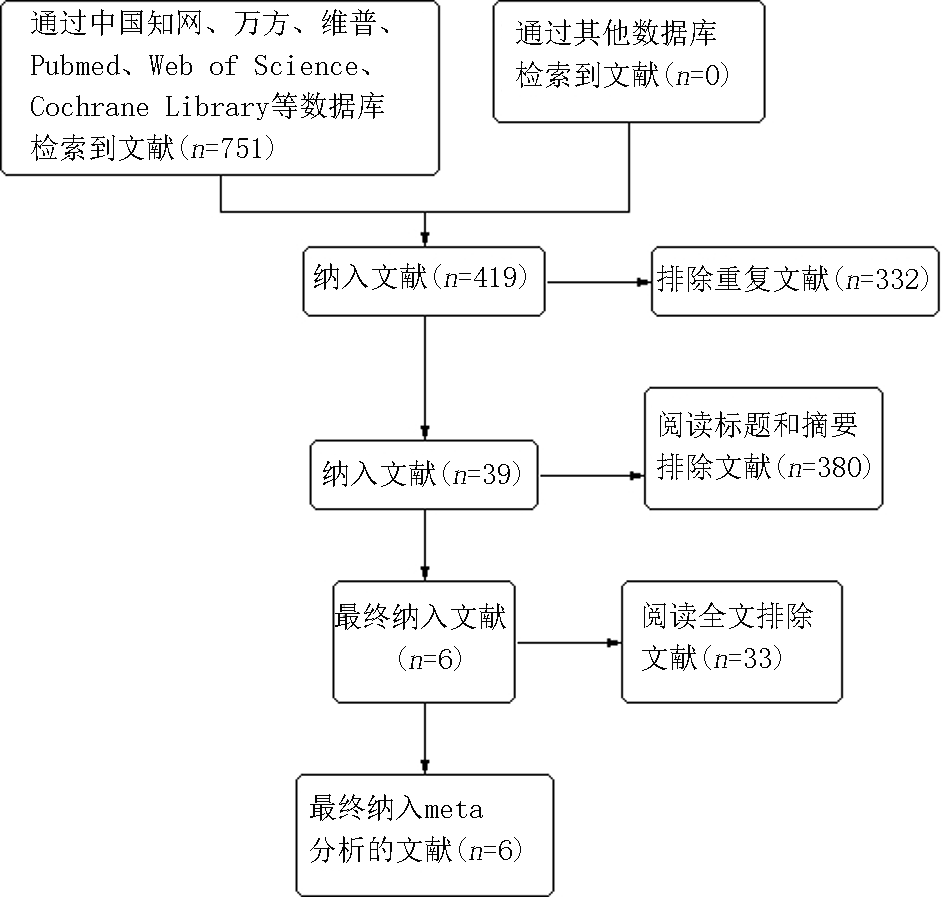
Clinical Focus ›› 2025, Vol. 40 ›› Issue (2): 101-106.doi: 10.3969/j.issn.1004-583X.2025.02.001
Association between D-dimer and the long-term prognosis of advanced cancer patients receiving PD-1/PD-L1 inhibitors: A meta-analysis
- 1. Yangzhou Center for Disease Control and Prevention,Yangzhou 225001,China
2. Department of Oncology, Affiliated Hospital of Yangzhou University,Yangzhou 225100,China
-
Received:2024-11-03Online:2025-02-20Published:2025-03-04 -
Contact:Liu Shenxiang E-mail:lsx810914@163.com
CLC Number:
Cite this article
Ye Qian, Liu Shenxiang. Association between D-dimer and the long-term prognosis of advanced cancer patients receiving PD-1/PD-L1 inhibitors: A meta-analysis[J]. Clinical Focus, 2025, 40(2): 101-106.
share this article
Tab.1 Basic characteristics of included literatures
| 第一作者 | 发表 年份 | 研究 类型 | 肿瘤 类型 | 患者 数量 | 年龄(岁, 中位数,范围) | PD-1/PD-L1 抑制剂类型 | D-二聚体 检测时间 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wei等[ | 2021 | 回顾性 | 晚期NSCLC | 64 | - | 帕博利珠单抗 | 治疗前、最佳缓解期、疾病进展期 | ||||||
| Zhang等[ | 2022 | 回顾性 | 晚期MSS/pM MR CRC | 110 | 53(22-81) | 信迪利单抗、卡瑞利珠单抗、特瑞普利单抗、替雷利珠单抗、帕博利珠单抗 | 治疗前 | ||||||
| Li等[ | 2022 | 回顾性 | 晚期NSCLC | 277 | 61(33-91) | - | 治疗前 | ||||||
| Chen等[ | 2023 | 回顾性 | 晚期或复发 NSCLC | 100 | D-二聚体<0.98 mg/L:63(57-68);E-D-二聚体≥0.98 mg/L:64(54.5-68) | 纳武利尤单抗、信迪利单抗、卡瑞利珠单抗、帕博利珠单抗 | 治疗前 | ||||||
| Sun等[ | 2024 | 回顾性 | 晚期NSCLC | 80 | 63(34-73) | 信迪利单抗、卡瑞利珠单抗、特瑞普利单抗 | 治疗前、最佳缓解期、疾病进展期 | ||||||
| Wu等[ | 2024 | 回顾性 | 晚期或转移性ESCC | 233 | - | - | 治疗前 | ||||||
| 第一作者 | D-二聚体 截断值(mg/L) | 生存 结局 | 单因素分析(HR,95%CI) | 多因素分析(HR,95%CI) | |||||||||
| OS | PFS | OS | PFS | ||||||||||
| Wei等[ | 1.51 | PFS | - | - | - | 1.72(0.59~5.00) | |||||||
| Zhang等[ | 0.30 | PFS | - | 1.71(1.09~2.70) | - | 1.28(0.80~2.04) | |||||||
| Li等[ | 0.50 | OS、PFS | 2.29(1.52~3.46) | 1.80(1.30~2.49) | 2.13(1.40~3.25) | 1.84(1.32~2.56) | |||||||
| Chen等[ | 0.98 | PFS | - | 7.37(2.53~21.51) | - | 14.09(3.26~60.84) | |||||||
| Sun等[ | - | OS | 1.00(1.00~1.00) | - | - | - | |||||||
| Wu等[ | 0.24 | OS、PFS | 1.90(1.42~2.53) | 1.51(1.15~1.98) | 2.05(1.52~2.76) | 1.52(1.15~2.03) | |||||||
Tab.1 Basic characteristics of included literatures
| 第一作者 | 发表 年份 | 研究 类型 | 肿瘤 类型 | 患者 数量 | 年龄(岁, 中位数,范围) | PD-1/PD-L1 抑制剂类型 | D-二聚体 检测时间 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wei等[ | 2021 | 回顾性 | 晚期NSCLC | 64 | - | 帕博利珠单抗 | 治疗前、最佳缓解期、疾病进展期 | ||||||
| Zhang等[ | 2022 | 回顾性 | 晚期MSS/pM MR CRC | 110 | 53(22-81) | 信迪利单抗、卡瑞利珠单抗、特瑞普利单抗、替雷利珠单抗、帕博利珠单抗 | 治疗前 | ||||||
| Li等[ | 2022 | 回顾性 | 晚期NSCLC | 277 | 61(33-91) | - | 治疗前 | ||||||
| Chen等[ | 2023 | 回顾性 | 晚期或复发 NSCLC | 100 | D-二聚体<0.98 mg/L:63(57-68);E-D-二聚体≥0.98 mg/L:64(54.5-68) | 纳武利尤单抗、信迪利单抗、卡瑞利珠单抗、帕博利珠单抗 | 治疗前 | ||||||
| Sun等[ | 2024 | 回顾性 | 晚期NSCLC | 80 | 63(34-73) | 信迪利单抗、卡瑞利珠单抗、特瑞普利单抗 | 治疗前、最佳缓解期、疾病进展期 | ||||||
| Wu等[ | 2024 | 回顾性 | 晚期或转移性ESCC | 233 | - | - | 治疗前 | ||||||
| 第一作者 | D-二聚体 截断值(mg/L) | 生存 结局 | 单因素分析(HR,95%CI) | 多因素分析(HR,95%CI) | |||||||||
| OS | PFS | OS | PFS | ||||||||||
| Wei等[ | 1.51 | PFS | - | - | - | 1.72(0.59~5.00) | |||||||
| Zhang等[ | 0.30 | PFS | - | 1.71(1.09~2.70) | - | 1.28(0.80~2.04) | |||||||
| Li等[ | 0.50 | OS、PFS | 2.29(1.52~3.46) | 1.80(1.30~2.49) | 2.13(1.40~3.25) | 1.84(1.32~2.56) | |||||||
| Chen等[ | 0.98 | PFS | - | 7.37(2.53~21.51) | - | 14.09(3.26~60.84) | |||||||
| Sun等[ | - | OS | 1.00(1.00~1.00) | - | - | - | |||||||
| Wu等[ | 0.24 | OS、PFS | 1.90(1.42~2.53) | 1.51(1.15~1.98) | 2.05(1.52~2.76) | 1.52(1.15~2.03) | |||||||
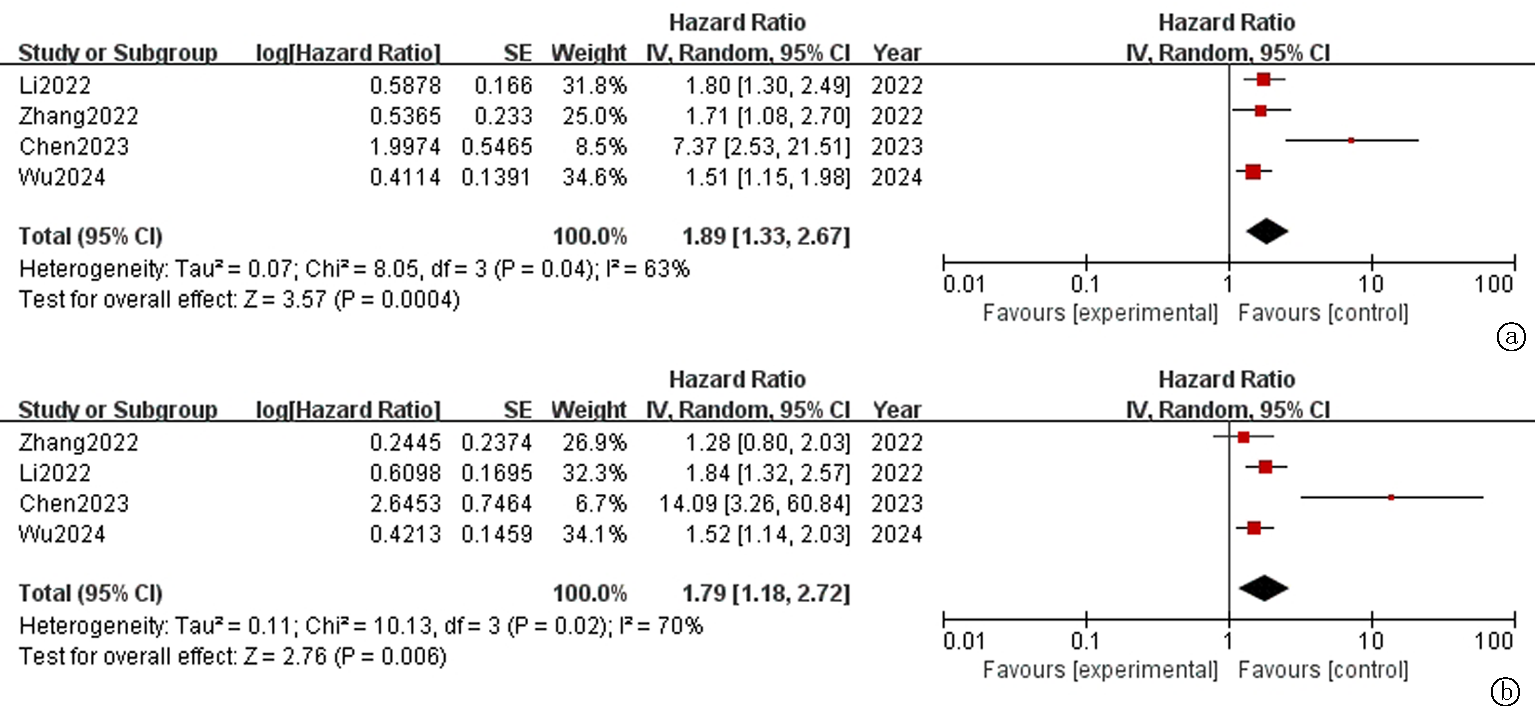
Fig.2 Forest plot of overall survival(OS) in advanced cancer patients receiving PD-1/PD-L1 inhibitors a. Univariate analysis; b. Multivariate analysis
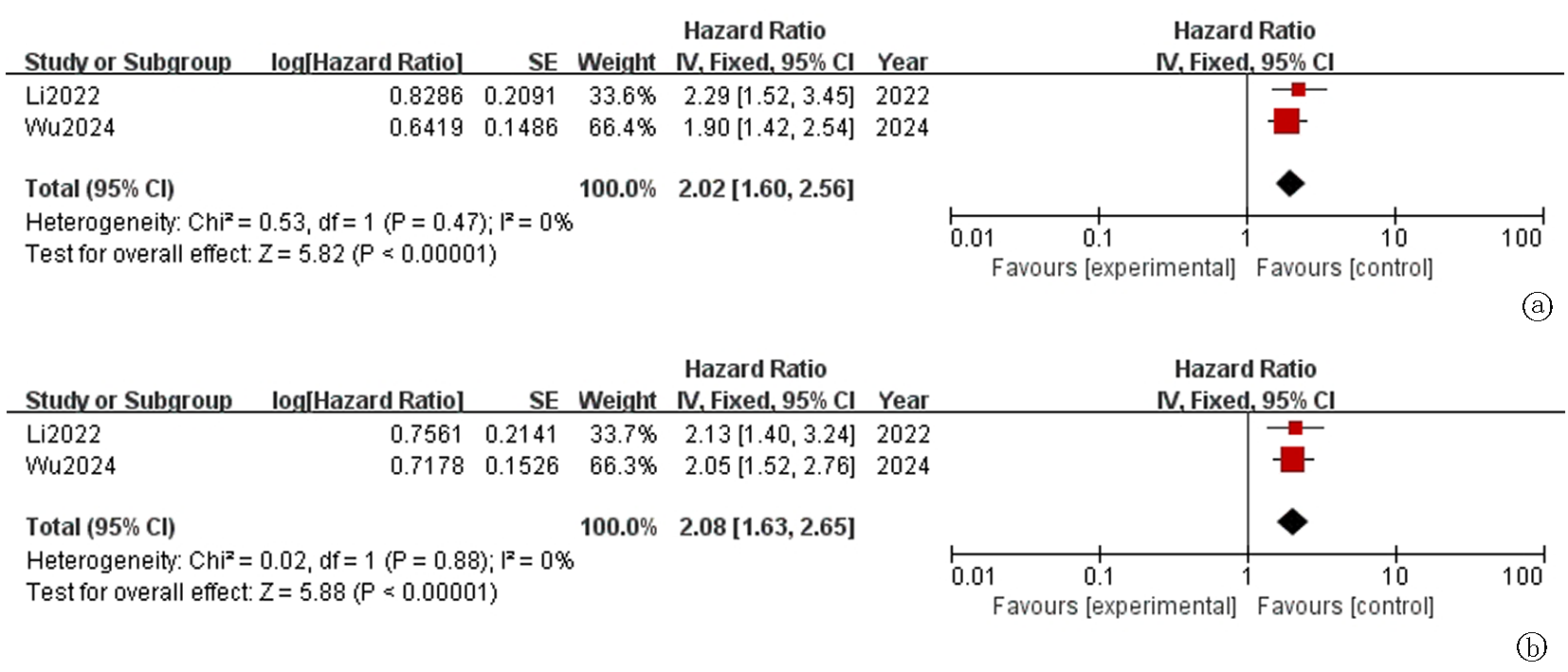
Fig.2 Forest plot of overall survival(OS) in advanced cancer patients receiving PD-1/PD-L1 inhibitors a. Univariate analysis; b. Multivariate analysis
Tab.2 PFS subgroup analysis
| 亚组 | 单因素分析 | 亚组 | 多因素分析 | ||||
|---|---|---|---|---|---|---|---|
| 文献数量 | HR(95%CI) | P | 文献数量 | HR(95%CI) | P | ||
| 肿瘤类型 | 肿瘤类型 | ||||||
| NSCLC | 2 | 3.31(0.84~13.00) | 0.09 | NSCLC | 2 | 4.47(0.62~32.34) | 0.14 |
| CRC | 1 | 1.71(1.08~2.70) | 0.02 | CRC | 1 | 1.28(0.80~2.03) | 0.30 |
| ESCC | 1 | 1.51(1.15~1.98) | 0.003 | ESCC | 1 | 1.52(1.14~2.03) | 0.004 |
| D-二聚体截断值(mg/L) | D-二聚体截断值(mg/L) | ||||||
| <0.5 | 2 | 1.56(1.23~1.97) | 0.0002 | <0.5 | 2 | 1.45(1.14~1.85) | 0.003 |
| ≥0.5 | 2 | 3.31(0.84~13.00) | 0.09 | ≥0.5 | 2 | 4.47(0.62~32.34) | 0.14 |
Tab.2 PFS subgroup analysis
| 亚组 | 单因素分析 | 亚组 | 多因素分析 | ||||
|---|---|---|---|---|---|---|---|
| 文献数量 | HR(95%CI) | P | 文献数量 | HR(95%CI) | P | ||
| 肿瘤类型 | 肿瘤类型 | ||||||
| NSCLC | 2 | 3.31(0.84~13.00) | 0.09 | NSCLC | 2 | 4.47(0.62~32.34) | 0.14 |
| CRC | 1 | 1.71(1.08~2.70) | 0.02 | CRC | 1 | 1.28(0.80~2.03) | 0.30 |
| ESCC | 1 | 1.51(1.15~1.98) | 0.003 | ESCC | 1 | 1.52(1.14~2.03) | 0.004 |
| D-二聚体截断值(mg/L) | D-二聚体截断值(mg/L) | ||||||
| <0.5 | 2 | 1.56(1.23~1.97) | 0.0002 | <0.5 | 2 | 1.45(1.14~1.85) | 0.003 |
| ≥0.5 | 2 | 3.31(0.84~13.00) | 0.09 | ≥0.5 | 2 | 4.47(0.62~32.34) | 0.14 |
| [1] | Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022[J]. CA Cancer J Clin, 2022, 72(1):7-33. |
| [2] |
Jiang Y, Zhao X, Fu J, et al. Progress and challenges in precise treatment of tumors with PD-1/PD-L1 blockade[J]. Front Immunol, 2020, 11:339.
doi: 10.3389/fimmu.2020.00339 pmid: 32226426 |
| [3] | Pang K, Shi ZD, Wei LY, et al. Research progress of therapeutic effects and drug resistance of immunotherapy based on PD-1/PD-L1 blockade[J]. Drug Resist Updat, 2023, 66:100907. |
| [4] |
Ma M, Cao R, Wang W, et al. The D-dimer level predicts the prognosis in patients with lung cancer: A systematic review and meta-analysis[J]. J Cardiothorac Surg, 2021, 16(1):243.
doi: 10.1186/s13019-021-01618-4 pmid: 34454552 |
| [5] | Shang X, Li X. D-dimer and the short-term prognosis of patients with subarachnoid hemorrhage: A meta-analysis[J]. Eur Neurol, 2024, 87(4):188-202. |
| [6] |
Li W, Tang Y, Song Y, et al. Prognostic role of pretreatment plasma d-dimer in patients with solid tumors: A systematic review and meta-analysis[J]. Cell Physiol Biochem, 2018, 45(4):1663-1676.
doi: 10.1159/000487734 pmid: 29490291 |
| [7] | Liu T, Tang LV. Incidence and risk factors of venous and arterial thromboembolism in immune checkpoint inhibitor therapy[J]. Curr Pharm Des, 2022, 28(36):2950-2952. |
| [8] |
Moik F, Chan WE, Wiedemann S, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy[J]. Blood, 2021, 137(12):1669-1678.
doi: 10.1182/blood.2020007878 pmid: 33067632 |
| [9] | Wei XY, Zhang CC, Zang FL, et al. Preliminary study on inflammatory markers for predicting the efficacy and prognosis of anti-PD-1 antibody treatment in patients with non-small cell lung cancer[J]. Chin J Clin Oncol, 2021, 48(11):547-551. |
| [10] | Zhang W, Zhang Z, Lou S, et al. Efficacy, safety and predictors of combined fruquintinib with programmed death-1 inhibitors for advanced microsatellite-stable colorectal cancer: A retrospective study[J]. Frontiers in oncology, 2022, 12:929342. |
| [11] |
Li X, Lu D, Zhang Z, et al. Prognostic value of plasma D-dimer levels in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: A retrospective study[J]. J Thorac Dis, 2022, 14(10):4125-4135.
doi: 10.21037/jtd-22-1363 pmid: 36389301 |
| [12] | Chen C, Yin H, Zhang Y, et al. Plasma D-dimer and interleukin-6 are associated with treatment response and progression-free survival in advanced NSCLC patients on anti-PD-1 therapy[J]. Cancer Med, 2023, 12(15):15831-15840. |
| [13] | Sun W, Yao XM, Wang PJ, et al. Exploration of prognostic factors and nomogram construction for advanced non-small cell lung cancer treated with immunotherapy based on hematologic indexes[J]. J Int Oncol, 2024, 51(3):143-150. |
| [14] | Wu Y, Liu X, Li H, et al. D-dimer levels predict the treatment efficacy and prognosis of esophageal squamous cell carcinoma treated with PD-1/PD-L1 inhibitors[J]. Int J Biol Markers, 2024, 39(3):209-216. |
| [15] | Wu B, Zhang G, Zhao X, et al. Association between D-dimer levels and clinicopathological characteristics of pancreatic cancer and its role in prognosis: A systematic review and meta-analysis[J]. Asian J Surg, 2024, 47(8):3417-3424. |
| [16] | Zhou YX, Yang ZM, Feng J, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer: A meta-analysis[J]. Tumour Biol, 2013, 34(6):3701-3704. |
| [17] | Alkhoder L, Salamoon M, Saifo M, et al. D-dimer as a predictive biomarker of response to chemotherapy in patients with metastatic breast cancer[J]. Biomark Insights, 2024, 19:11772719241290704. |
| [18] | Singh AK, Malviya R. Coagulation and inflammation in cancer: Limitations and prospects for treatment[J]. Biochim Biophys Acta Rev Cancer, 2022, 1877(3):188727. |
| [19] | Kawashima S, Togashi Y. Resistance to immune checkpoint inhibitors and the tumor microenvironment[J]. Exp Dermatol, 2023, 32(3):240-249. |
| [20] | Kleinegris MC, ten Cate H, ten Cate-Hoek AJ. D-dimer as a marker for cardiovascular and arterial thrombotic events in patients with peripheral arterial disease. A systematic review[J]. Thromb Haemost, 2013, 110(2):233-243. |
| [21] | Zhang W, Zhang Z, Lou S, et al. Efficacy, safety and predictors of combined fruquintinib with programmed death-1 inhibitors for advanced microsatellite-stable colorectal cancer: A retrospective study[J]. Front Oncol, 2022, 12:929342. |
| [22] |
Chen C, Li J, Li J, et al. Application of an elevated plasma D-dimer cut-off value improves prognosis prediction of advanced non-small cell lung cancer[J]. Ann Transl Med, 2020, 8(18):1153.
doi: 10.21037/atm-20-5947 pmid: 33241002 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||