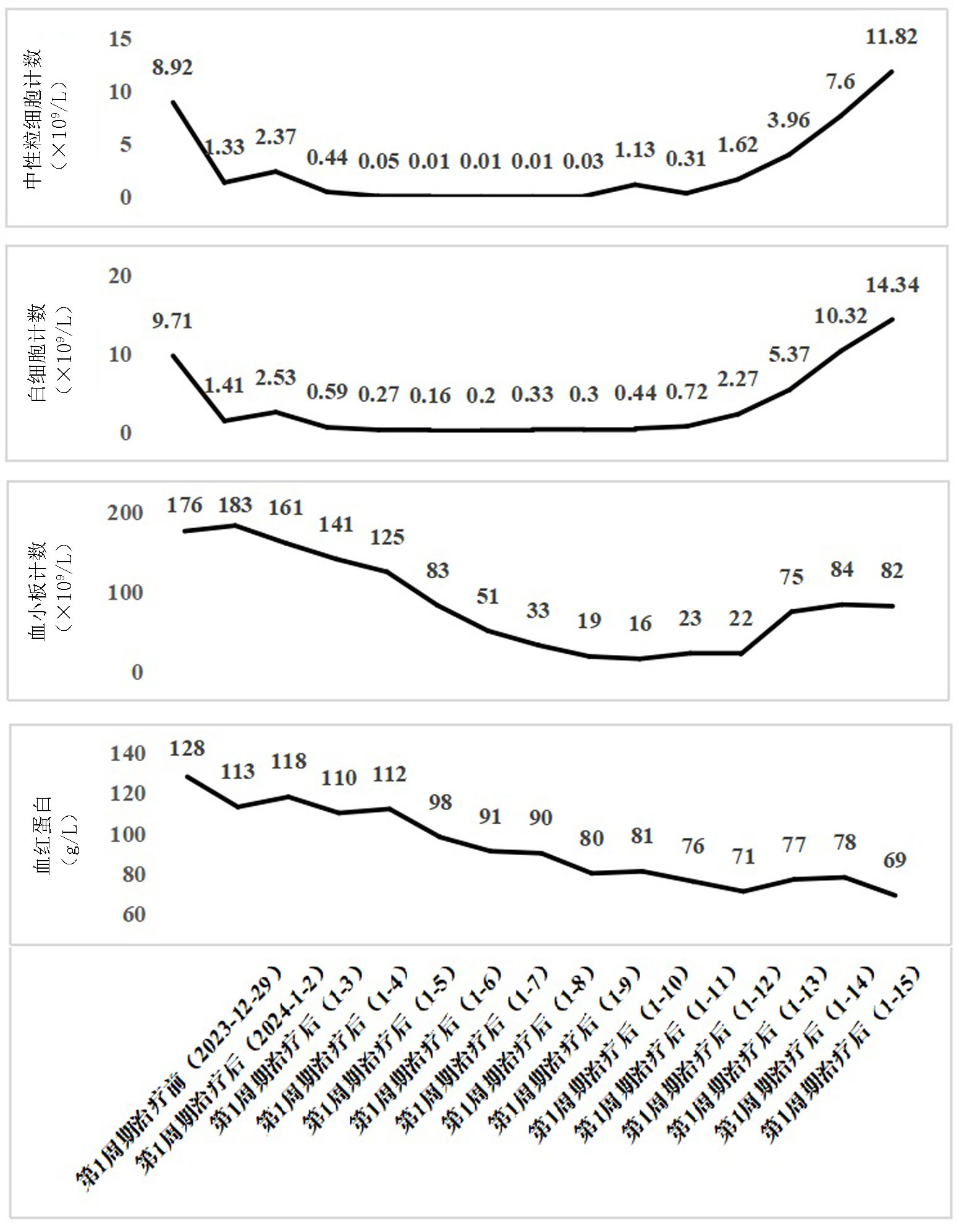
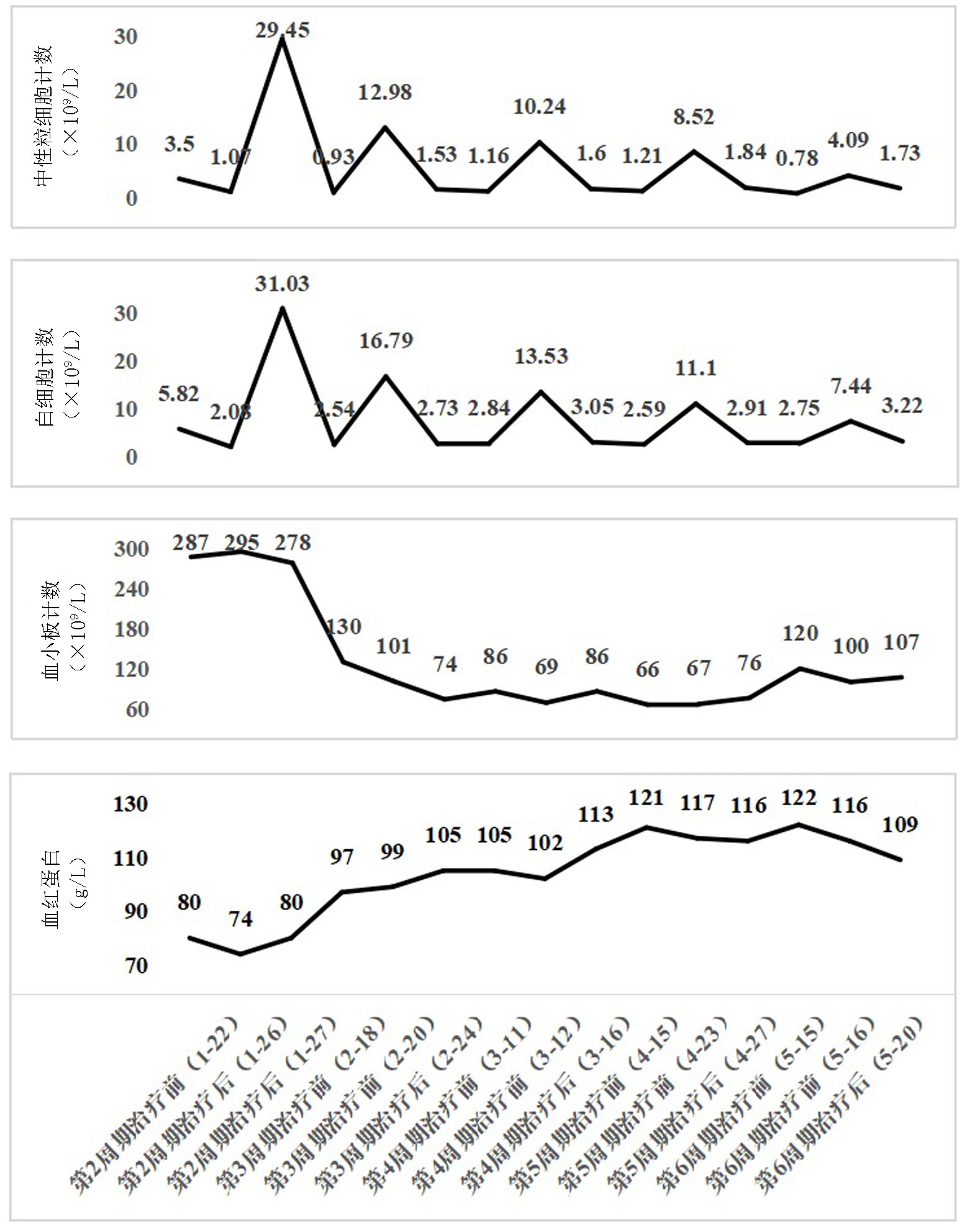
Clinical Focus ›› 2025, Vol. 40 ›› Issue (4): 355-359.doi: 10.3969/j.issn.1004-583X.2025.04.011
Previous Articles Next Articles
Trilaciclib combined with chemotherapy in the treatment of extensive-stage small cell lung cancer: A case report and literature review
- Department of Respiratory and Critical Care Medicine,the First Affiliated Hospital of Xi'an Jiaotong University,Xi'an 710061,China
-
Received:2024-11-04Online:2025-04-20Published:2025-04-17 -
Contact:Li Manxiang E-mail:manxiangli@hotmail.com
CLC Number:
Cite this article
Wang Yinfeng, Li Manxiang. Trilaciclib combined with chemotherapy in the treatment of extensive-stage small cell lung cancer: A case report and literature review[J]. Clinical Focus, 2025, 40(4): 355-359.
share this article
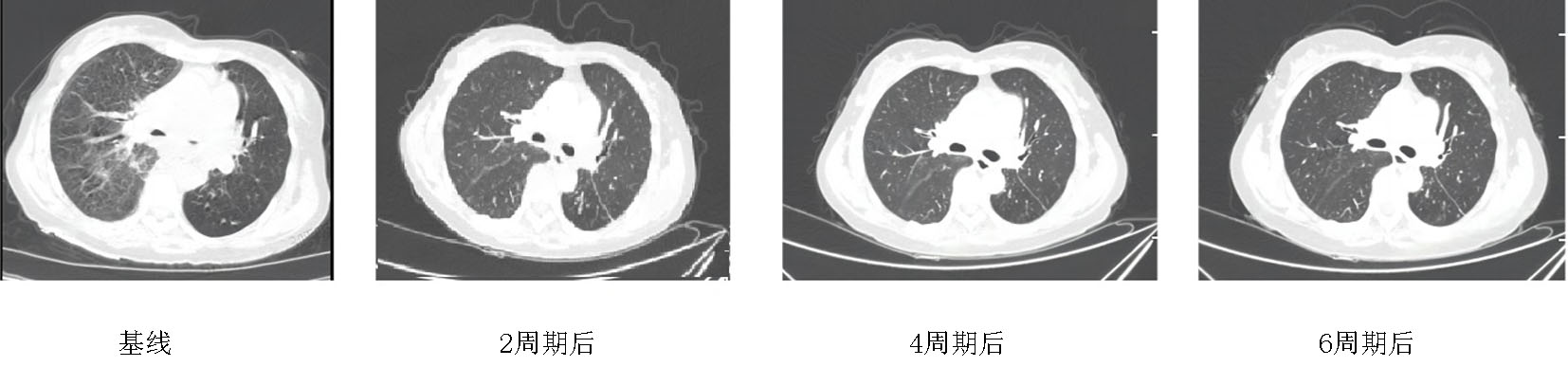
Fig. 3 Chest enhanced CT: After 2, 4 and 6 cycles of treatment, the lesions were significantly reduced, and the efficacy was evaluated as partial remission
| 周期(时间) | 用药方案 | 抗肿瘤疗效 | 骨髓抑制分级 |
|---|---|---|---|
| 第1周期(2023-12-30) | 依托泊苷145 mg(d1~3)+顺铂40 mg(d1~2)+顺铂30 mg(d3) | - | Ⅳ级 |
| 第2周期(2024-01-23) | 曲拉西利300mg(d1~2)+依托泊苷110 mg(d1~3)+顺铂40 mg(d1~2) | PR | Ⅲ级 |
| 第3周期(2024-02-21) | 曲拉西利300 mg(d1~2)+依托泊苷110mg(d1~3)+顺铂40 mg(d1~2) | - | Ⅱ级 |
| 第4周期(2024-03-13) | 曲拉西利300 mg(d1~2)+依托泊苷110mg(d1~3)+顺铂40 mg(d1~2) | PR | Ⅰ级 |
| 第5周期(2024-04-24) | 曲拉西利300mg(d1~2)+依托泊苷110 mg(d1~3)+顺铂40 mg(d1~2) | - | Ⅱ级 |
| 第6周期(2024-05-17) | 曲拉西利300 mg(d1~2)+依托泊苷110mg(d1~3)+顺铂40 mg(d1~2) | PR | Ⅰ级 |
Tab. 1 Efficacy and myelosuppression grade in each cycle
| 周期(时间) | 用药方案 | 抗肿瘤疗效 | 骨髓抑制分级 |
|---|---|---|---|
| 第1周期(2023-12-30) | 依托泊苷145 mg(d1~3)+顺铂40 mg(d1~2)+顺铂30 mg(d3) | - | Ⅳ级 |
| 第2周期(2024-01-23) | 曲拉西利300mg(d1~2)+依托泊苷110 mg(d1~3)+顺铂40 mg(d1~2) | PR | Ⅲ级 |
| 第3周期(2024-02-21) | 曲拉西利300 mg(d1~2)+依托泊苷110mg(d1~3)+顺铂40 mg(d1~2) | - | Ⅱ级 |
| 第4周期(2024-03-13) | 曲拉西利300 mg(d1~2)+依托泊苷110mg(d1~3)+顺铂40 mg(d1~2) | PR | Ⅰ级 |
| 第5周期(2024-04-24) | 曲拉西利300mg(d1~2)+依托泊苷110 mg(d1~3)+顺铂40 mg(d1~2) | - | Ⅱ级 |
| 第6周期(2024-05-17) | 曲拉西利300 mg(d1~2)+依托泊苷110mg(d1~3)+顺铂40 mg(d1~2) | PR | Ⅰ级 |
| [1] | Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022[J]. J Natl Cancer Centr, 2024, 4(1): 47-53. |
| [2] |
Oronsky B, Reid TR, Oronsky A, et al. What's new in SCLC? A review[J]. Neoplasia, 2017, 19(10): 842-847.
doi: S1476-5586(17)30270-1 pmid: 28888101 |
| [3] |
Saltos A, Shafique M, Chiappori A. Update on the biology, management, and treatment of small cell lung cancer (SCLC)[J]. Front Oncol, 2020, 10:1074.
doi: 10.3389/fonc.2020.01074 pmid: 32766139 |
| [4] |
Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: Results from an online survey of patients with solid tumors[J]. Adv Ther, 2020, 37(8): 3606-3618.
doi: 10.1007/s12325-020-01419-6 pmid: 32642965 |
| [5] | Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy[J]. Oncology (Williston Park), 2015, 29(4): 282-294. |
| [6] | Aapro M, Beguin Y, Bokemeyer C, et al. Management of anaemia and iron deficiency in patients with cancer: ESMO clinical practice guidelines[J]. Ann Oncol, 2018, 29(Suppl 4): iv96-iv110. |
| [7] | Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO clinical practice guidelines[J]. Ann Oncol, 2016, 27(Suppl 5): v111-v118. |
| [8] | 秦叔逵, 马军. 中国临床肿瘤学会(CSCO)肿瘤放化疗相关中性粒细胞减少症规范化管理指南(2021)[J]. 临床肿瘤学杂志, 2021, 26(7):638-648. |
| [9] | 马军, 王杰军, 张力, 等. 肿瘤相关性贫血临床实践指南(2015-2016版)[J]. 中国实用内科杂志, 2016, 36(S1):1-21. |
| [10] | 史艳侠, 邢镨元, 张俊, 等. 中国肿瘤化疗相关性血小板减少症专家诊疗共识(2019版)[J]. 中国肿瘤临床, 2019, 46(18):923-929. |
| [11] | 吴育锋, 余利蒙, 赵玉华, 等. 小细胞肺癌化疗相关骨髓抑制的研究进展[J]. 中国新药杂志, 2023, 32(23):2347-2353. |
| [12] | Dhillon S. Trilaciclib: First approval[J]. Drugs, 2021, 81(7): 867-874. |
| [13] | 抗肿瘤药物引起骨髓抑制中西医结合诊治专家共识[J]. 临床肿瘤学杂志, 2021, 26(11):1020-1027. |
| [14] | Banys-Paluchowski M, Krawczyk N, Paluchowski P. Cyclin-dependent kinase 4/6 inhibitors: What have we learnt across studies, therapy situations and substances[J]. Curr Opin Obstet Gynecol, 2019, 31(1):56-66. |
| [15] | Daniel D, Kuchava V, Bondarenko I, et al. Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: A multicentre, randomised, double-blind, placebo-controlled phase II trial[J]. Int J Cancer, 2021, 148(10): 2557-2570. |
| [16] |
Hart LL, Ferrarotto R, Andric ZG, et al. Myelopreservation with trilaciclib in patients receiving topotecan for small cell lung cancer: Results from a randomized, double-blind, placebo-controlled phase II study[J]. Adv Ther, 2021, 38(1): 350-365.
doi: 10.1007/s12325-020-01538-0 pmid: 33123968 |
| [17] |
Weiss JM, Csoszi T, Maglakelidze M, et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: A phase Ib/randomized phase II trial[J]. Ann Oncol, 2019, 30(10): 1613-1621.
doi: S0923-7534(19)60981-6 pmid: 31987452 |
| [18] | 徐华, 李封, 王娟, 等. 曲拉西利联合免疫联合化疗一线治疗晚期非小细胞肺癌1例及文献分析[J]. 中国新药杂志, 2024, 33(11):1126-1132. |
| [19] | 严京泽, 陈慧, 葛小林, 等. 曲拉西利联合免疫联合化疗治疗晚期食管癌1例及文献分析[J]. 中国新药杂志, 2023, 32(23):2370-2374. |
| [20] | 袁静, 卢卫平, 肖国栋, 等. 曲拉西利联合拓扑替康二线治疗广泛期小细胞肺癌1例及文献分析[J]. 中国新药杂志, 2023, 32(23):2360-2365. |
| [21] | Tan AR, Wright GS, Thummala AR, et al. Trilaciclib prior to chemotherapy in patients with metastatic triple-negative breast cancer: Final efficacy and subgroup analysis from a randomized phase II study[J]. Clin Cancer Res, 2022, 28(4):629-636. |
| [22] | Lai AY, Sorrentino JA, Dragnev KH, et al. CDK4/6 inhibition enhances antitumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy[J]. J Immunother Cancer, 2020, 8(2):e000847. |
| [23] |
Deng J, Wang ES, Jenkins RW, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation[J]. Cancer Discov, 2018, 8(2): 216-233.
doi: 10.1158/2159-8290.CD-17-0915 pmid: 29101163 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||