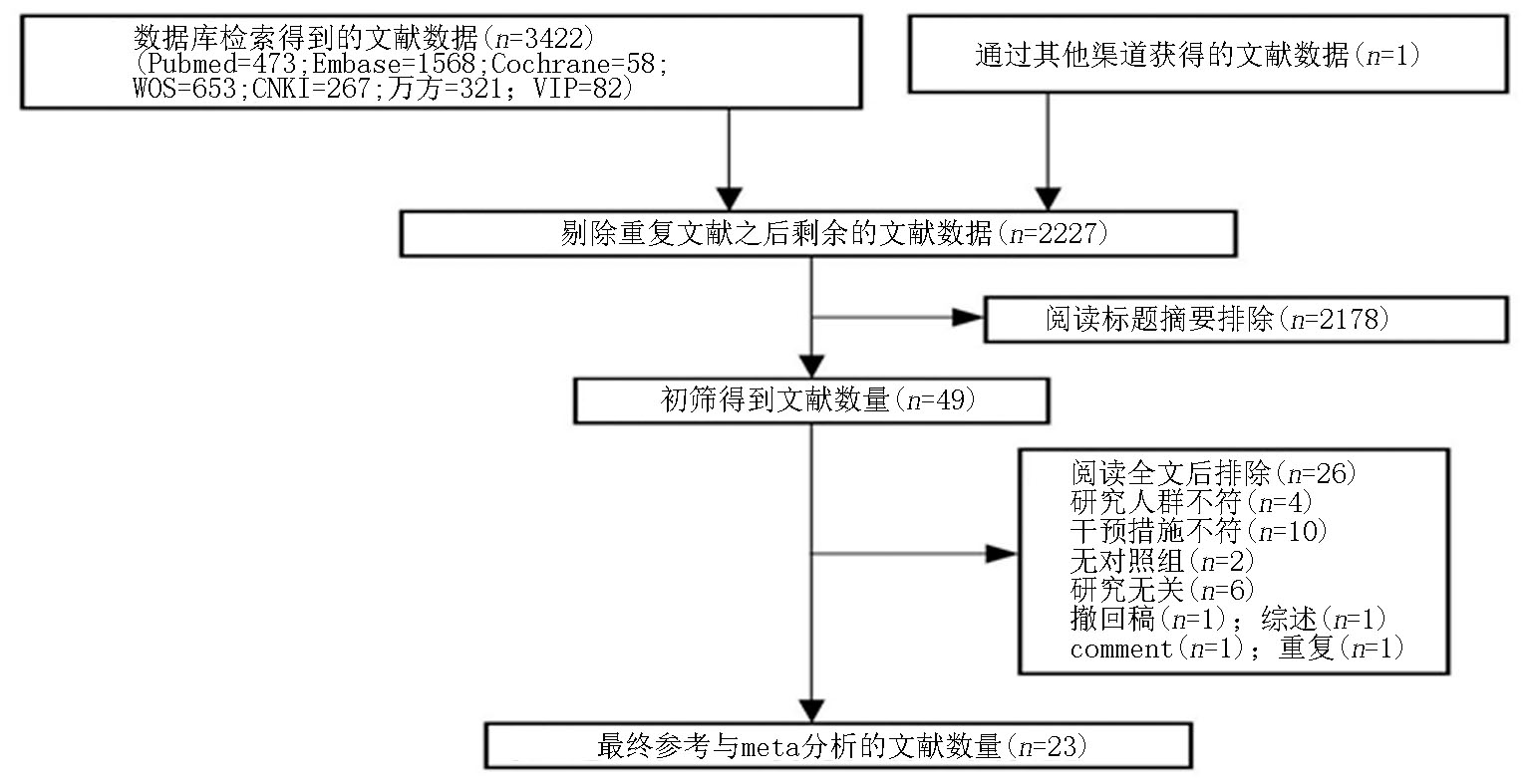
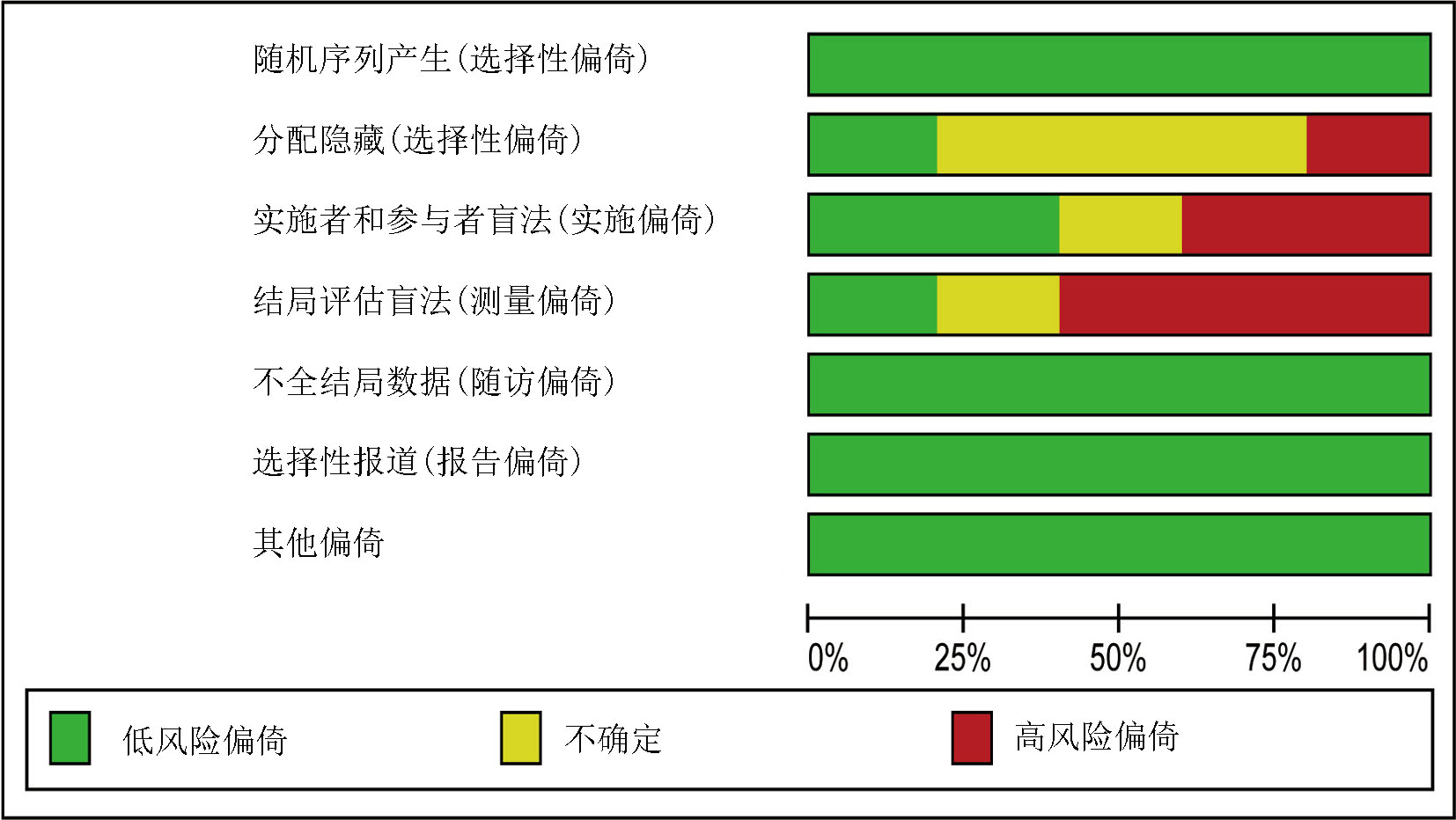
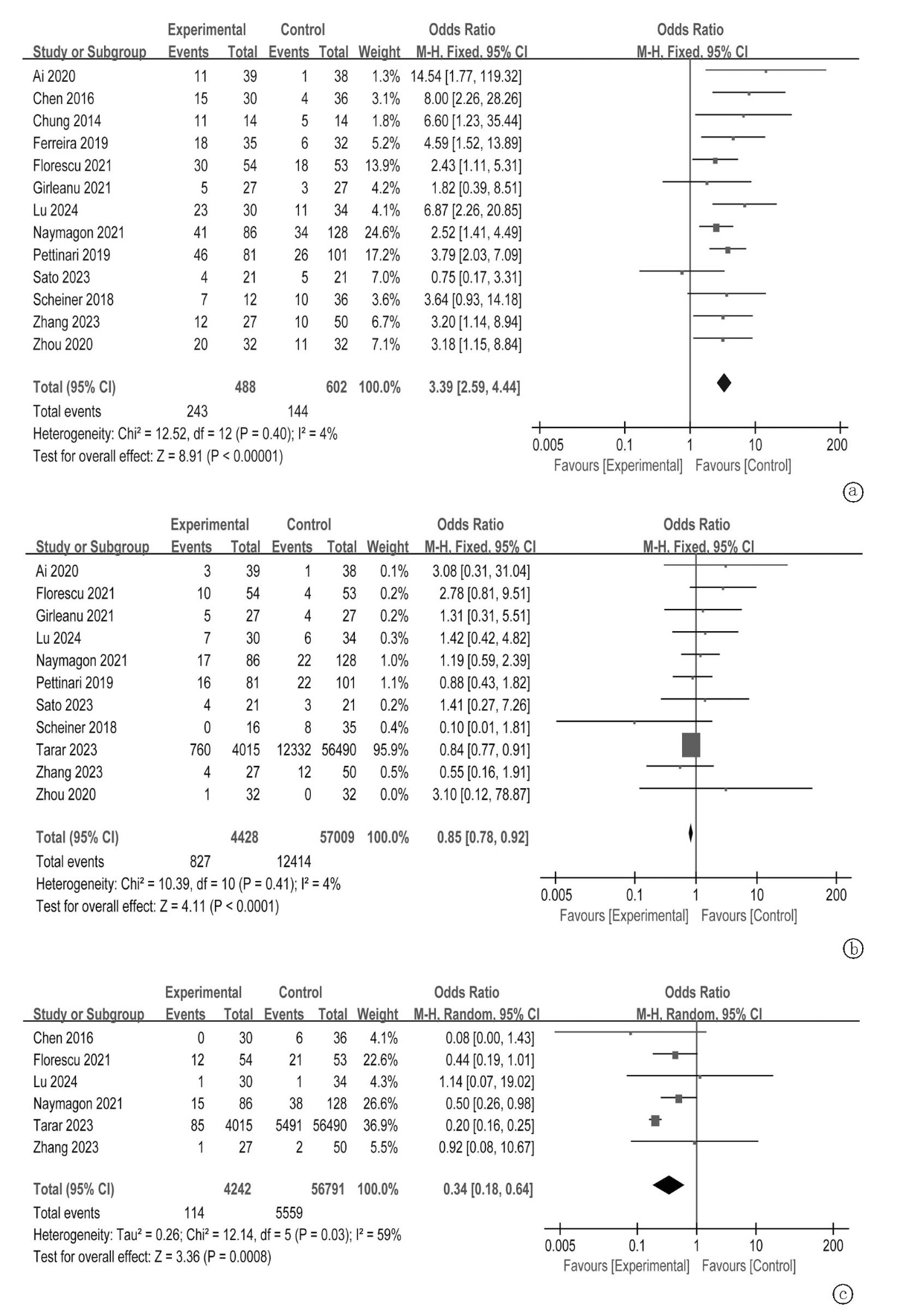
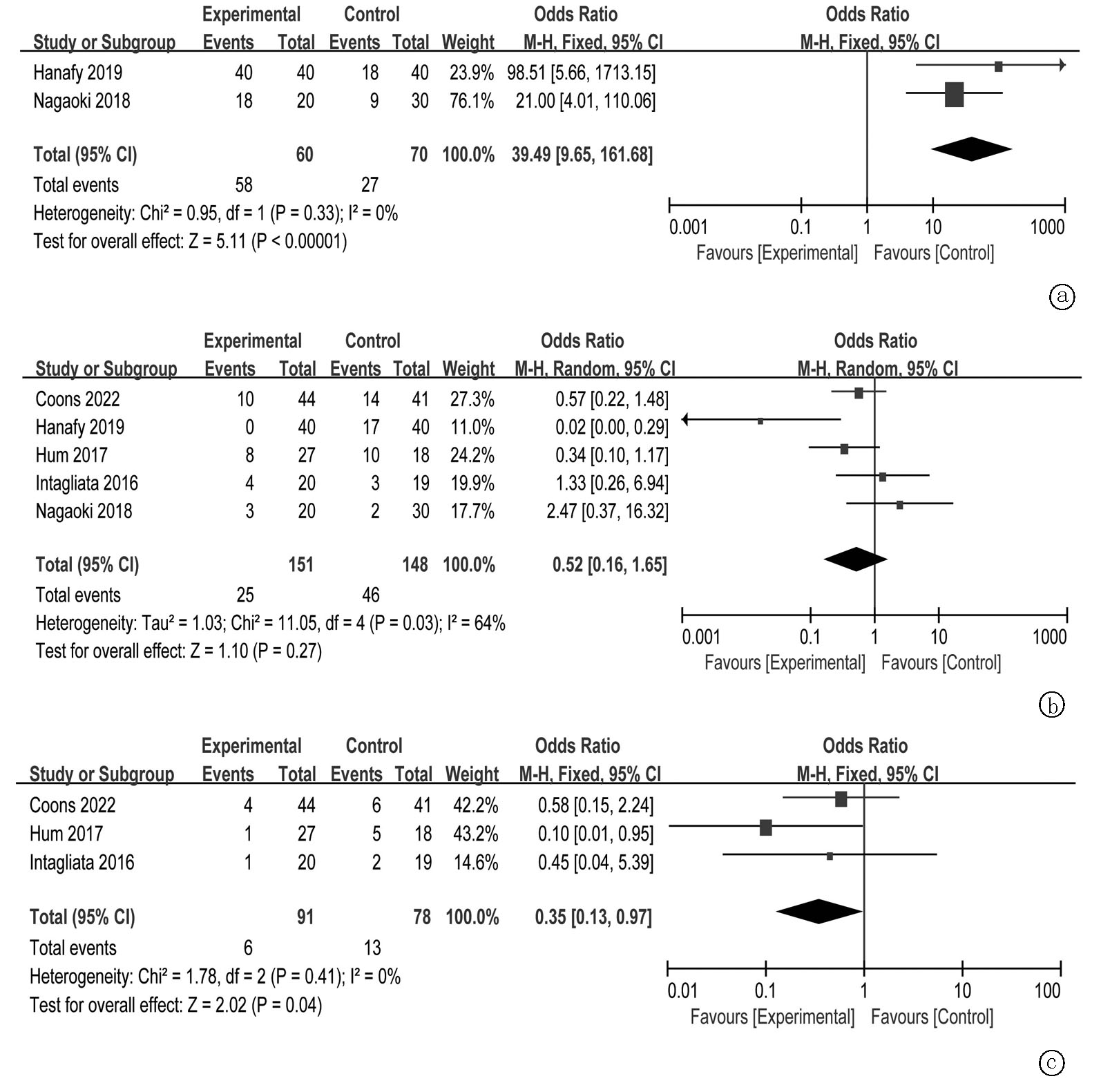
Clinical Focus ›› 2025, Vol. 40 ›› Issue (4): 293-303.doi: 10.3969/j.issn.1004-583X.2025.04.001
Efficacy and safety of anticoagulant therapy in patients with cirrhosis: A meta-analysis
Su Rui1,2, Wang Cunkai1, Wang Dingxin1, Cai Conghui1, Zhang Jian1, Hou Hongtao1, Bai Yun1( )
)
- 1.Department of Geriatric Gastroenterology,Hebei General Hospital,Shijiazhuang 050071,China
2.Graduate Academy,Hebei Medical University,Shijiazhuang 050017,China
-
Received:2024-11-25Online:2025-04-20Published:2025-04-17 -
Contact:Bai Yun E-mail:luckycloud@126.com
CLC Number:
Cite this article
Su Rui, Wang Cunkai, Wang Dingxin, Cai Conghui, Zhang Jian, Hou Hongtao, Bai Yun. Efficacy and safety of anticoagulant therapy in patients with cirrhosis: A meta-analysis[J]. Clinical Focus, 2025, 40(4): 293-303.
share this article
Tab.1 Basic characteristics of included studies
| 作者 | 年份 | 国家 | 随访时间 | 抗凝方案 | 试验组人数 /对照组人数 | 性别 (男/女) | 年龄(岁) (试验组/对照组) | 肝硬化病因 (病毒/酒精/其他) | Child-Pugh 分级(A/B/C) |
|---|---|---|---|---|---|---|---|---|---|
| 抗凝治疗 | |||||||||
| Chung[ | 2014 | 韩国 | 至少3月 | 华法林 | 14/14 | 21/7 | 59.4±12.0 58.7±13.2 | 19/7/2 | 13/14/1 |
| Naymagon[ | 2021 | 美国 | 27(12-48)月 | 华法林、依诺肝素、利伐沙班、阿哌沙班、达比加群 | 86/128 | 142/72 | 60(54-66) | 80/43/91 | 52/99/63 |
| Zhang[ | 2023 | 中国 | 26(13-44)月 | 华法林、利伐沙班、达比加群、低分子肝素 | 27/50 | 44/33 | 59.0±13.0 60.4±12.3 | 24/18/35 | 32/37/8 |
| Tarar[ | 2023 | 美国 | NA | NA | 4015/56490 | 38721/21784 | 59.8(58.9-60.6) 59.9(59.7-60.1) | NA | NA |
| Ai[ | 2020 | 中国 | 6月 | 利伐沙班或达比加群 | 40/40 | 50/30 | 56.1±16.1 52.3±19.4 | NA | NA |
| Zhou[ | 2020 | 中国 | 6月 | 1个月那屈肝素钙,5个月华法林 | 32/32 | 42/22 | 55±9 53±10 | 47/8/9 | NA |
| Chen[ | 2016 | 中国 | 33.2±29.2月 25.9±23月 | 华法林 | 30/36 | 47/19 | 44.97±12.3 47.86±10.6 | 42/3/21 | NA |
| Lu[ | 2024 | 中国 | 1年 | 华法林 | 30/34 | 22/42 | 55(24-69) | 45/7/12 | 42/21/1 |
| Pettinari[ | 2019 | 意大利 | 19(3-94)月 | 低分子肝素、磺达肝癸钠、VKA | 81/101 | 130/52 | 57.8±11.2 | 84/47/51 | 80/78/24 |
| Florescu[ | 2021 | 罗马尼亚 | 32(3-109)月 | 依诺肝素、VKA | 54/53 | 54/53 | 53(23-73) 55.65(25-75) | 70/23/14 | 27/77/3 |
| Scheiner[ | 2018 | 奥地利 | 44.1(14.0-79.1)月 | 低分子肝素、苯丙香豆素 | 12/36 | 32/19 | 52.9±12.5 | 6/24/21 | 14/19/18 |
| Ferreira[ | 2019 | 葡萄牙 | 25(1-146)月 | 华法林、低分子肝素 | 35/32 | 53/27 | 60±9 | 11/45/24 | 21/34/25 |
| Sato[ | 2023 | 日本 | NA | DOAC、华法林钾、肝素 | 21/21 | 31/11 | 68(64-71) 69(63-77) | 7/25/10 | NA |
| Girleanu[ | 2021 | 罗马尼亚 | 6月 | 依诺肝素 | 27/27 | NA | 58.91±8.98 | 35%酒精 | 0/28/26 |
| DOAC vs传统抗凝药 | |||||||||
| Coons[ | 2022 | 美国 | 最长1年 | DOAC VS华法林 | 44/41 | 54/31 | 67.2±9 63.6±10.5 | 32/20/33 | 27/41/17 |
| Hanafy[ | 2019 | 埃及 | NA | 利伐沙班VS华法林 | 40/40 | 67/13 | 43.2±3.8 | HCV | NA |
| Hum[ | 2017 | 美国 | NA | DOAC VS VKA或低分子肝素 | 27/18 | 32/13 | 61(26-90) 58(34-80) | 16/11/21 | 18/21/6 |
| Intagliata[ | 2016 | 美国 | NA | DOAC VS华法林或低分子肝素 | 20/19 | 22/17 | 57(50-64) 60(55-64) | 6/5/28 | 18/21/0 |
| Nagaoki[ | 2018 | 日本 | 6月 | 依度沙班VS华法林 | 20/30 | 30/20 | 68(23-83) | 33病变 | 29/16/5 |
| 预防性抗凝治疗 | |||||||||
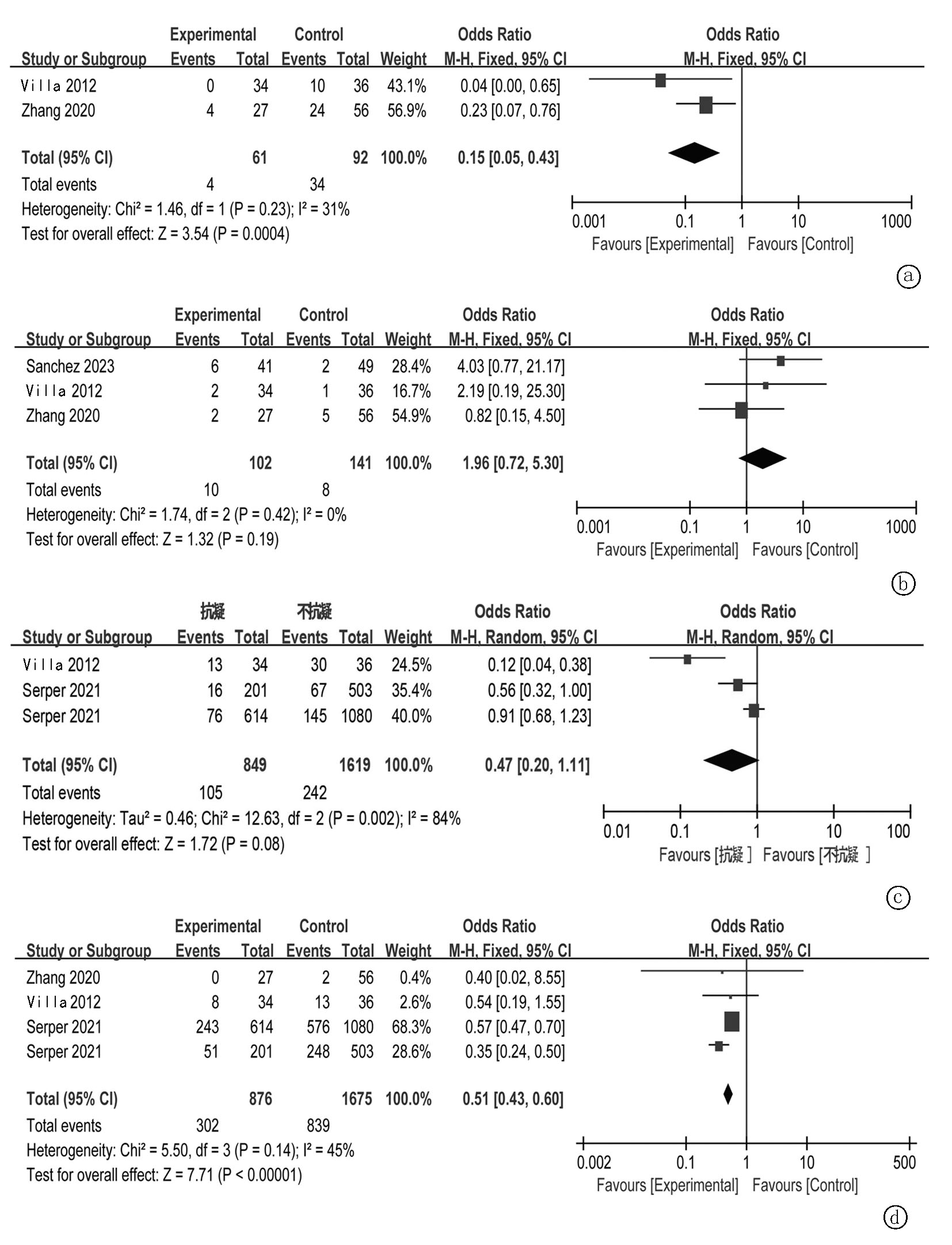
| Zhang[ | 2020 | 中国 | 23.8±9.9月 25.0±10.4月 | 华法林 | 27/56 | 61/22 | 51.7±14.2 51.4±10.5 | 50/15/18 | 22/53/8 |
| Villa[ | 2012 | 意大利 | 89±57周 58±37周 | 依诺肝素 | 34/36 | 51/19 | 56±5 57±7 | 32/24/14 | 0/64/6 |
| Sanchez[ | 2023 | 西班牙 | 10.1(0.4-24)月 | 利伐沙班 | 41/49 | 74/16 | 58.1±7.6 | 86.7%酒精 | NA |
| Serper[ | 2021 | 美国 | 4.6年 | 华法林 | 614/1080 | 1667/27 | 64.6±7.5 64.2±8.4 | 1222HCV/酒精, 472其他 | 1217/461/16 |
| Serper[ | 2021 | 美国 | 4.6年 | DOAC | 201/503 | 697/7 | 64.0±7.7 64.3±8.4 | 509HCV/酒精, 195其他 | 639/65/0 |
Tab.1 Basic characteristics of included studies
| 作者 | 年份 | 国家 | 随访时间 | 抗凝方案 | 试验组人数 /对照组人数 | 性别 (男/女) | 年龄(岁) (试验组/对照组) | 肝硬化病因 (病毒/酒精/其他) | Child-Pugh 分级(A/B/C) |
|---|---|---|---|---|---|---|---|---|---|
| 抗凝治疗 | |||||||||
| Chung[ | 2014 | 韩国 | 至少3月 | 华法林 | 14/14 | 21/7 | 59.4±12.0 58.7±13.2 | 19/7/2 | 13/14/1 |
| Naymagon[ | 2021 | 美国 | 27(12-48)月 | 华法林、依诺肝素、利伐沙班、阿哌沙班、达比加群 | 86/128 | 142/72 | 60(54-66) | 80/43/91 | 52/99/63 |
| Zhang[ | 2023 | 中国 | 26(13-44)月 | 华法林、利伐沙班、达比加群、低分子肝素 | 27/50 | 44/33 | 59.0±13.0 60.4±12.3 | 24/18/35 | 32/37/8 |
| Tarar[ | 2023 | 美国 | NA | NA | 4015/56490 | 38721/21784 | 59.8(58.9-60.6) 59.9(59.7-60.1) | NA | NA |
| Ai[ | 2020 | 中国 | 6月 | 利伐沙班或达比加群 | 40/40 | 50/30 | 56.1±16.1 52.3±19.4 | NA | NA |
| Zhou[ | 2020 | 中国 | 6月 | 1个月那屈肝素钙,5个月华法林 | 32/32 | 42/22 | 55±9 53±10 | 47/8/9 | NA |
| Chen[ | 2016 | 中国 | 33.2±29.2月 25.9±23月 | 华法林 | 30/36 | 47/19 | 44.97±12.3 47.86±10.6 | 42/3/21 | NA |
| Lu[ | 2024 | 中国 | 1年 | 华法林 | 30/34 | 22/42 | 55(24-69) | 45/7/12 | 42/21/1 |
| Pettinari[ | 2019 | 意大利 | 19(3-94)月 | 低分子肝素、磺达肝癸钠、VKA | 81/101 | 130/52 | 57.8±11.2 | 84/47/51 | 80/78/24 |
| Florescu[ | 2021 | 罗马尼亚 | 32(3-109)月 | 依诺肝素、VKA | 54/53 | 54/53 | 53(23-73) 55.65(25-75) | 70/23/14 | 27/77/3 |
| Scheiner[ | 2018 | 奥地利 | 44.1(14.0-79.1)月 | 低分子肝素、苯丙香豆素 | 12/36 | 32/19 | 52.9±12.5 | 6/24/21 | 14/19/18 |
| Ferreira[ | 2019 | 葡萄牙 | 25(1-146)月 | 华法林、低分子肝素 | 35/32 | 53/27 | 60±9 | 11/45/24 | 21/34/25 |
| Sato[ | 2023 | 日本 | NA | DOAC、华法林钾、肝素 | 21/21 | 31/11 | 68(64-71) 69(63-77) | 7/25/10 | NA |
| Girleanu[ | 2021 | 罗马尼亚 | 6月 | 依诺肝素 | 27/27 | NA | 58.91±8.98 | 35%酒精 | 0/28/26 |
| DOAC vs传统抗凝药 | |||||||||
| Coons[ | 2022 | 美国 | 最长1年 | DOAC VS华法林 | 44/41 | 54/31 | 67.2±9 63.6±10.5 | 32/20/33 | 27/41/17 |
| Hanafy[ | 2019 | 埃及 | NA | 利伐沙班VS华法林 | 40/40 | 67/13 | 43.2±3.8 | HCV | NA |
| Hum[ | 2017 | 美国 | NA | DOAC VS VKA或低分子肝素 | 27/18 | 32/13 | 61(26-90) 58(34-80) | 16/11/21 | 18/21/6 |
| Intagliata[ | 2016 | 美国 | NA | DOAC VS华法林或低分子肝素 | 20/19 | 22/17 | 57(50-64) 60(55-64) | 6/5/28 | 18/21/0 |
| Nagaoki[ | 2018 | 日本 | 6月 | 依度沙班VS华法林 | 20/30 | 30/20 | 68(23-83) | 33病变 | 29/16/5 |
| 预防性抗凝治疗 | |||||||||
| Zhang[ | 2020 | 中国 | 23.8±9.9月 25.0±10.4月 | 华法林 | 27/56 | 61/22 | 51.7±14.2 51.4±10.5 | 50/15/18 | 22/53/8 |
| Villa[ | 2012 | 意大利 | 89±57周 58±37周 | 依诺肝素 | 34/36 | 51/19 | 56±5 57±7 | 32/24/14 | 0/64/6 |
| Sanchez[ | 2023 | 西班牙 | 10.1(0.4-24)月 | 利伐沙班 | 41/49 | 74/16 | 58.1±7.6 | 86.7%酒精 | NA |
| Serper[ | 2021 | 美国 | 4.6年 | 华法林 | 614/1080 | 1667/27 | 64.6±7.5 64.2±8.4 | 1222HCV/酒精, 472其他 | 1217/461/16 |
| Serper[ | 2021 | 美国 | 4.6年 | DOAC | 201/503 | 697/7 | 64.0±7.7 64.3±8.4 | 509HCV/酒精, 195其他 | 639/65/0 |
Tab.2 The quality of studies (NOS scores)
| 研究 | Selection | Comparability | Outcome | Stars |
|---|---|---|---|---|
| Chung 2014[ | 4 | 2 | 2 | 8 |
| Naymagon 2021[ | 4 | 2 | 3 | 9 |
| Zhang 2023[ | 4 | 1 | 3 | 8 |
| Tarar 2023[ | 4 | 2 | 3 | 9 |
| Ai 2020[ | 4 | 2 | 3 | 9 |
| Chen 2016[ | 4 | 2 | 3 | 9 |
| Pettinari 2019[ | 4 | 1 | 3 | 8 |
| Florescu 2021[ | 4 | 2 | 3 | 9 |
| Scheiner 2018[ | 4 | 1 | 2 | 7 |
| Ferreira 2019[ | 4 | 2 | 2 | 8 |
| Sato 2023[ | 4 | 2 | 2 | 8 |
| Girleanu 2021[ | 2 | 2 | 2 | 6 |
| Coons 2022[ | 4 | 2 | 2 | 8 |
| Hum 2017[ | 4 | 2 | 2 | 8 |
| Intagliata 2016[ | 4 | 2 | 3 | 9 |
| Nagaoki 2018[ | 4 | 2 | 3 | 9 |
| Zhang 2020[ | 4 | 2 | 3 | 9 |
| Serper 2021[ | 4 | 2 | 3 | 9 |
Tab.2 The quality of studies (NOS scores)
| 研究 | Selection | Comparability | Outcome | Stars |
|---|---|---|---|---|
| Chung 2014[ | 4 | 2 | 2 | 8 |
| Naymagon 2021[ | 4 | 2 | 3 | 9 |
| Zhang 2023[ | 4 | 1 | 3 | 8 |
| Tarar 2023[ | 4 | 2 | 3 | 9 |
| Ai 2020[ | 4 | 2 | 3 | 9 |
| Chen 2016[ | 4 | 2 | 3 | 9 |
| Pettinari 2019[ | 4 | 1 | 3 | 8 |
| Florescu 2021[ | 4 | 2 | 3 | 9 |
| Scheiner 2018[ | 4 | 1 | 2 | 7 |
| Ferreira 2019[ | 4 | 2 | 2 | 8 |
| Sato 2023[ | 4 | 2 | 2 | 8 |
| Girleanu 2021[ | 2 | 2 | 2 | 6 |
| Coons 2022[ | 4 | 2 | 2 | 8 |
| Hum 2017[ | 4 | 2 | 2 | 8 |
| Intagliata 2016[ | 4 | 2 | 3 | 9 |
| Nagaoki 2018[ | 4 | 2 | 3 | 9 |
| Zhang 2020[ | 4 | 2 | 3 | 9 |
| Serper 2021[ | 4 | 2 | 3 | 9 |
| [1] |
Senzolo M, Garcia-Pagan JC. A major research gap: The use of anticoagulants in cirrhosis[J]. J Hepatol, 2023, 79(6):1566-1570.
doi: 10.1016/j.jhep.2023.06.001 pmid: 37302580 |
| [2] | 祁兴顺, 杨玲. 肝硬化门静脉血栓管理专家共识(2020年,上海)[J]. 临床肝胆病杂志, 2020, 36(12):2667-2674. |
| [3] | EASL Clinical Practice Guidelines. Vascular diseases of the liver[J]. J Hepatol, 2016, 64(1):179-202. |
| [4] |
de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII-Renewing consensus in portal hypertension[J]. J Hepatol, 2022, 76(4):959-974.
doi: 10.1016/j.jhep.2021.12.022 pmid: 35120736 |
| [5] | 李媛. 肝硬化预防性抗凝治疗的临床研究进展[D]. 南昌: 南昌大学, 2017. |
| [6] |
Chung JW, Kim GH, Lee JH, et al. Safety, efficacy, and response predictors of anticoagulation for the treatment of nonmalignant portal-vein thrombosis in patients with cirrhosis: A propensity score matching analysis[J]. Clin Mol Hepatol, 2014, 20(4): 384-391.
doi: 10.3350/cmh.2014.20.4.384 pmid: 25548745 |
| [7] | Naymagon L, Tremblay D, Zubizarreta N, et al.Safety, efficacy, and long-term outcomes of anticoagulation in cirrhotic portal vein thrombosis[J]. Dig Dis Sci, 2021, 66(10):3619-3629. |
| [8] |
Zhang Z, Zhao Y, Li D, et al. Safety, efficacy and prognosis of anticoagulant therapy for portal vein thrombosis in cirrhosis: A retrospective cohort study[J]. Thromb J, 2023, 21(1):13.
doi: 10.1186/s12959-023-00454-x pmid: 36717831 |
| [9] | Tarar ZI, Farooq U, Kamal F, et al. Safety of anticoagulation use for treatment of portal vein thrombosis in liver cirrhosis and its effect on hospital-based outcomes: An insight from a US nationwide database[J]. Postgrad Med J, 2023, 99:715-723. |
| [10] | Ai MH, Dong WG, Tan XP, et al. Efficacy and safety study of direct-acting oral anticoagulants for the treatment of chronic portal vein thrombosis in patients with liver cirrhosis[J]. Eur J Gastroenterol Hepatol, 2020, 32(10):1395-1400. |
| [11] | Zhou T, Sun X, Zhou T, et al. Efficacy and safety of nadroparin calcium-warfarin sequential anticoagulation in portal vein thrombosis in cirrhotic patients: A randomized controlled trial[J]. Clin Transl Gastroenterol, 2020, 11(9): e00228. |
| [12] | Chen H, Liu L, Qi X, et al. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis[J]. Eur J Gastroenterol Hepatol, 2016, 28(1): 82-89. |
| [13] | Lu S, Chen J, Zhang R, et al. Comparative effectiveness of warfarin in cirrhotic patients with non-symptomatic portal vein thrombosis: A multicenter, randomized controlled trial[J]. Expert Rev Gastroent, 2024, 18(1-3): 5-12. |
| [14] |
Pettinari I, Vukotic R, Stefanescu H, et al. Clinical impact and safety of anticoagulants for portal vein thrombosis in cirrhosis[J]. Am J Gastroenterol, 2019, 114(2): 258-266.
doi: 10.1038/s41395-018-0421-0 pmid: 30538290 |
| [15] | Florescu MM, Costache A, Iacob SM, et al. Anticoagulation therapy for portal vein thrombosis in patients with cirrhosis in a tertiary center experience[J]. J Gastrointest Liver, 2021, 30(3): 374-379. |
| [16] |
Scheiner B, Stammet PR, Pokorny S, et al. Anticoagulation in non-malignant portal vein thrombosis is safe and improves hepatic function[J]. Wien Klin Wochenschr, 2018, 130(13-14): 446-455.
doi: 10.1007/s00508-018-1351-y pmid: 29916054 |
| [17] |
Noronha Ferreira C, Reis D, Cortez-Pinto H, et al. Anticoagulation in cirrhosis and portal vein thrombosis is safe and improves prognosis in advanced cirrhosis[J]. Digest Dis Sci, 2019, 64(9): 2671-2683.
doi: 10.1007/s10620-019-05572-z pmid: 30852769 |
| [18] | Sato A, Watanabe S, Iseki M, et al. Anticoagulation against portal vein thrombosis reduces mortality and liver cirrhosis-related complications: A propensity score-matched study[J]. Hepatol Res, 2023, 53(11): 1096-1104. |
| [19] | Girleanu I, Huiban L, Stanciu C, et al. Anticoagulant treatment in cirrhotic portal vein thrombosis-how wide can we open the window?[J]. J Gastrointest Liver, 2021, 30(4): 530-531. |
| [20] | Coons EM, Staubes BA, Casey AL, et al. Direct oral anticoagulants versus warfarin for treatment of thrombosis or atrial fibrillation in patients with cirrhosis: A retrospective cohort study[J]. Ann Pharmacother, 2022, 56:533-540. |
| [21] | Hanafy AS, Abd-Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis[J]. Vascul Pharmacol, 2019, 113:86-91. |
| [22] |
Hum J, Shatzel JJ, Jou JH, et al. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis[J]. Eur J Haematol, 2017, 98(4): 393-397.
doi: 10.1111/ejh.12844 pmid: 28009449 |
| [23] |
Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation[J]. Digest Dis Sci, 2016, 61(6): 1721-1727.
doi: 10.1007/s10620-015-4012-2 pmid: 26725062 |
| [24] |
Nagaoki Y, Aikata H, Daijyo K, et al. Efficacy and safety of edoxaban for treatment of portal vein thrombosis following danaparoid sodium in patients with liver cirrhosis[J]. Hepatol Res, 2018, 48(1):51-58.
doi: 10.1111/hepr.12895 pmid: 28342265 |
| [25] | Zhang L, Huan H, Tong H, et al. Warfarin prevented de novo portal vein thrombosis after transjugular intrahepatic portosystemic shunt: A retrospective study[J]. Medicine(Baltimore), 2020, 99(2): e18737. |
| [26] |
Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis[J]. Gastroenterology, 2012, 143(5): 1253-1260.e4.
doi: S0016-5085(12)01011-6 pmid: 22819864 |
| [27] | Sanchez AP, Turon F, Martinez J, et al. Rivaroxaban improves survival and decompensation in cirrhotic patients with moderate liver dysfunction. Double-blind, placebo-controlled trial[J]. J Hepatol, 2023, 78:S2-S3. |
| [28] | Serper M, Weinberg EM, Cohen JB, et al. Mortality and hepatic decompensation in patients with cirrhosis and atrial fibrillation treated with anticoagulation[J]. Hepatology, 2021, 73(1):219-232. |
| [29] |
Naymagon L. Venous thrombosis of the liver: Current and emerging concepts in management[J]. Transl Res, 2020, 225:54-69.
doi: S1931-5244(20)30075-X pmid: 32407789 |
| [30] |
Intagliata NM, Henry ZH, Shah N, et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding[J]. Liver Int, 2014, 34(1): 26-32.
doi: 10.1111/liv.12211 pmid: 23758818 |
| [31] | Dhar A, Anstee QM, Cobbold JF, et al. Anticoagulation for liver fibrosis: A pilot study in hepatitis c infected patients: 1706[J]. Hepatology, 2010, 52: 1133 A. |
| [32] | Seijo S, Garcia-Pagan JC. Anticoagulation in cirrhosis: Ready... set... wait![J]. Hepatology, 2013, 58(3):1175-1176. |
| [33] |
Ng CH, Tan DJH, Nistala KRY, et al. A network meta-analysis of direct oral anticoagulants for portal vein thrombosis in cirrhosis[J]. Hepatol Int, 2021, 15(5):1196-1206.
doi: 10.1007/s12072-021-10247-x pmid: 34417718 |
| [34] | Koh JH, Liew ZH, Ng GK, et al. Efficacy and safety of direct oral anticoagulants versus vitamin K antagonist for portal vein thrombosis in cirrhosis: A systematic review and meta-analysis[J]. Dig Liver Dis, 2022, 54(1):56-62. |
| [35] | Niu C, Zhang J, Himal K, et al. Impact of anticoagulation therapy on outcomes in patients with cirrhosis and portal vein thrombosis: A large-scale retrospective cohort study[J]. Thromb Res, 2024,241:109103. |
| [36] |
Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials[J]. Lancet, 2014, 383(9921):955-962.
doi: 10.1016/S0140-6736(13)62343-0 pmid: 24315724 |
| [37] | Huang ZC, Li CQ, Liu XY, et al. Efficacy and safety of direct oral anticoagulants in patients with atrial fibrillation and liver disease: A meta-analysis and systematic review[J]. Cardiovasc Drugs Ther, 2021, 35(6):1205-1215. |
| [38] | Li Z, Xu W, Wang L, et al. Risk of bleeding in liver cirrhosis receiving direct oral anticoagulants: A systematic review and meta-analysis[J]. Thromb Haemost, 2023, 123(11):1072-1088. |
| [39] |
Weinberg EM, Palecki J, Reddy KR. Direct-acting oral anticoagulants (DOACs) in cirrhosis and cirrhosisassociated portal vein thrombosis[J]. Semin Liver Dis, 2019, 39(2):195-208.
doi: 10.1055/s-0039-1679934 pmid: 30978730 |
| [40] |
Intagliata NM, Northup PG. Anticoagulant therapy in patients with cirrhosis[J]. Semin Thromb Hemost, 2015, 41(5):514-519.
doi: 10.1055/s-0035-1550436 pmid: 26049069 |
| [41] | Cerini F, Martinez Gonzalez J, Puente Á, et al. Impact of anticoagulant therapy on upper gastrointestinal bleeding (UGI) in patients with liver cirrhosis. Results from a retrospective multicentric case-control study[J]. J Hepatol, 2014, 60S(1):S8. |
| [42] |
Wang L, Guo X, Xu X, et al. Anticoagulation favors thrombus recanalization and survival in patients with liver cirrhosis and portal vein thrombosis: Results of a meta-analysis[J]. Adv Ther, 2021, 38(1):495-520.
doi: 10.1007/s12325-020-01550-4 pmid: 33155180 |
| [43] | Ghazaleh S, Beran A, Aburayyan K, et al. Efficacy and safety of anticoagulation in non-malignant portal vein thrombosis in patients with liver cirrhosis: A systematic review and meta-analysis[J]. Ann Gastroenterol, 2021, 34(1):104-110. |
| [44] | Gao Y, Liu H, Tang F, et al. Efficacy and safety of anticoagulants in liver cirrhosis patients with portal vein thrombosis: A meta-analysis[J]. Clin Res Hepatol Gastroenterol, 2021, 45(2):101649. |
| [45] |
Yao W, Feng Y, Liu T, et al. Rivaroxaban versus low-molecular weight heparin plus warfarin prevents portal vein system thrombosis after splenectomy and pericardial devascularization: A randomized clinical trial[J]. Excli J, 2021, 20:537-549.
doi: 10.17179/excli2020-3120 pmid: 33883982 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||